The pervasive chemistry of metal–organic frameworks
See: https://pubs.rsc.org/en/journals/journalissues/cs#!issueid=cs038005&type=current&issnprint=0306-0012
Jeffrey R. Longa and Omar M. Yaghib
aDepartment of Chemistry, University of California, Berkeley, California 94720-1460, USA. E-mail: jrlong@berkeley.edu; Fax: +1 510 643 3546; Tel: +1 510 642 0860
bCenter for Reticular Chemistry, Department of Chemistry and Biochemistry, University of California, Los Angeles, Los Angeles, CA 90095, USA. E-mail: yaghi@chem.ucla.edu; Fax: +1 310 206 5891; Tel: +1 310 206 0398
 Jeffrey R. Long Jeffrey R. Long | Jeffrey R. Long was born in Rolla, Missouri (USA), in 1969. He received a Bachelor’s Degree in Chemistry from Cornell University in 1991 and a PhD in Chemistry from Harvard University in 1995. Following postdoctoral work at Harvard and the University of California, Berkeley, he joined the faculty in Chemistry at Berkeley in 1997. His research involves the synthesis of new inorganic clusters and solids with emphasis on magnetic and microporous materials. |
 Omar M. Yaghi Omar M. Yaghi | Omar M. Yaghi was born in Amman, Jordan (1965). He received his PhD from the University of Illinois-Urbana (1990) with Professor Walter G. Klemperer. He was an NSF Postdoctoral Fellow at Harvard University with Professor Richard H. Holm (1990–1992). He is currently Christopher S. Foote Professor in the Department of Chemistry and Biochemistry at UCLA. He directs the Center for Reticular Chemistry. He has established several research programs dealing with the reticular synthesis of discrete polyhedra and extended frameworks from organic and inorganic building blocks. |
The chemistry of metal–organic frameworks (MOFs) is developing at an extraordinary pace, with an exponential growth in the number of research papers and reviews appearing in the chemical literature (Fig. 1). It seems as though at least one article in almost every issue of every chemistry journal is now reporting on some aspect of these new materials. This is so much so that one might say that MOF chemistry is in a ‘runaway’ mode, in that it is leading to an abundance of breakthroughs and discoveries rarely accomplished in such a brief period of time. What is the excitement about? Why has this area captured the attention and imagination of so many? And, most importantly, what is so intrinsically different about MOF chemistry that causes it to be ubiquitous in the literature?
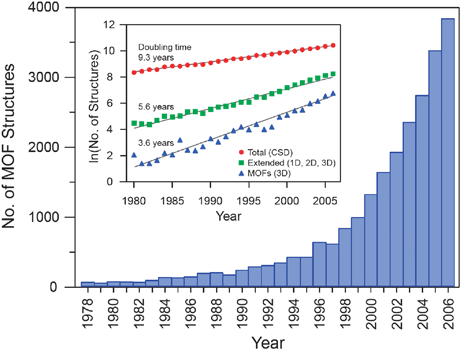 | ||
| Fig. 1 Number of metal–organic framework (MOF) structures reported in the Cambridge Structural Database (CSD) from 1978 through 2006. The bar graph illustrates the recent dramatic increase in the number of reports, while the inset shows the natural log of the number of structures as a function of time, indicating the extraordinarily short doubling time for MOF structures compared to the total number of structures archived in the database. |
The purpose of this themed issue is to provide some answers to these questions, while informing readers about a selection of topics in the field that are currently the subject of intense research. It suffices, however, to make the following observations regarding the fast-track development of MOF chemistry. It is now a matter of routine to use solution chemistry to link inorganic ‘joints’ with organic ‘struts’ by strong bonds to make robust and often porous MOFs featuring extended network architectures (Yaghi et al. DOI: 10.1039/b817735j; Zaworotko et al. DOI: 10.1039/b807086p). These materials are crystalline, so we know exactly where the atoms are and how they are connected, and the measured properties of the system can be correlated with the structure. More significantly, because the chemistry of the MOF components—i.e., of the individual organic molecules and the metal coordination sites—is so highly developed, we can have great confidence in our ability to formulate and execute a plan of how to produce a related material with specific changes in chemical functionality and metrics. Consequently, it is possible for one to first formulate a hypothesis of what structural attributes should give rise to the best performance for a specific application, and then to synthesize a variety of MOFs that test the hypothesis, and ultimately, by reiterating this process, to achieve truly exceptional properties. In this way, MOFs have been generated that exhibit the highest known surface areas, the lowest crystal densities, a high hydrogen storage capacity (Long et al. DOI: 10.1039/b802256a; Goddard et al. DOI: 10.1039/b802430h), selective heterogeneous catalysis (Hupp et al. DOI: 10.1039/b807080f; Lin et al. DOI: 10.1039/b807083k), magnetic ordering (Kurmoo, DOI: 10.1039/b804757j), channels for regulating polymerizations (Kitagawa et al. DOI: 10.1039/b802583p), selective carbon dioxide capture (Zhou et al. DOI: 10.1039/b802426j; Düren et al. DOI: 10.1039/b803498m; Shimizu et al. DOI: 10.1039/b802423p), and guest-dependent luminescence (Allendorf, Bauer et al. DOI: 10.1039/b802352m). Moreover, the drive for utilizing these highly-tunable materials in applications has led to development of new chemical methodologies for functionalizing their internal surfaces (Wang and Cohen, DOI: 10.1039/b802258p), and for obtaining them in flexible (Férey and Serre, DOI: 10.1039/b804302g), thin film (Fischer et al. DOI: 10.1039/b805038b), and nanoparticle forms (Mirkin et al. DOI: 10.1039/b807085g). Thus, the stage is set for this new class of materials to have the diversity, multiplicity, and exceptional properties that are required to solve some of our most pressing societal problems, including for example the creation and deployment of clean, sustainable sources of energy. Their widespread implementation will be significantly aided by the simple methods that have been developed to scale up the production of certain MOFs to ton-quantities (Czaja et al. DOI: 10.1039/b804680h).
On a fundamental level, MOFs combine chemistry and geometry to produce technology-generating properties in a way that is rarely experienced in science. These aspects are contributing to both the intellectual and the practical foundations of the field. The vast expanse of possibilities that MOF chemistry offers has without doubt allowed many colleagues from around the world to emerge as important leaders for their own unique contributions. This issue showcases only a small fraction of these contributions, and yet presents a diverse range of exciting recent developments in the field.
Source… https://pubs.rsc.org/en/journals/journalissues/cs#!issueid=cs038005&type=current&issnprint=0306-0012